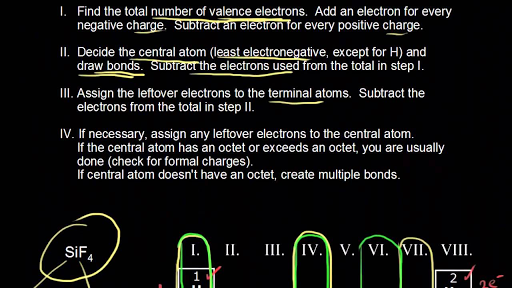
Covalent Lewis Dot Structure Calculator
Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice. Lewis Structures are important to learn because they help us predict: the shape of a molecule. How the molecule might react with other molecules. The physical properties of the molecule (like boiling point, surface tension, etc.). A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Electrons exist outside of an atom‘s nucleus and are found in principal energy levels that contain only up to a specific number of electrons. Get the free 'Lewis Structure Finder' widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram Alpha. Drawing the Lewis Structure for Cl 2 CO. Viewing Notes: The Lewis structure for Cl 2 CO requires you to place Carbon in the center of the structure since it is the most electronegative. You'll need a double bond between the Carbon and Oxygen atoms to acheive full outer shells for the atoms while still only using 24 valence electrons. Chapter 6 – Lewis Structures of Covalent Compounds Rules for Writing Lewis Structures: NASL Method The octet rule alone does not let us write Lewis structures. We still need to know how to place the electrons around the bonded atoms. The NASL method is an easier format for doing this. Steps for the NASL Method 1.) Select a reasonable skeletal.
| Writing Lewis Structures by Trial and Error | A Step-By-Step Approach to Writing Lewis Structures | Drawing Skeleton Structures |
| Molecules that Contain Too Many or Not Enough Electrons | Resonance Hybrids | Formal Charge |
Writing Lewis Structures by Trial and Error
The Lewis structure of a compound can be generated by trial and error. We start by writing symbols that contain the correct number of valence electrons for the atoms in the molecule. We then combine electrons to form covalent bonds until we come up with a Lewis structure in which all of the elements (with the exception of the hydrogen atoms) have an octet of valence electrons.
Example: Let's apply the trial and error approach to generating the Lewis structure of carbon dioxide, CO2. We start by determining the number of valence electrons on each atom from the electron configurations of the elements. Carbon has four valence electrons, and oxygen has six.
C: [He] 2s2 2p2
O: [He] 2s2 2p4
We can symbolize this information as shown at the top of the figure below. We now combine one electron from each atom to form covalent bonds between the atoms. When this is done, each oxygen atom has a total of seven valence electrons and the carbon atom has a total of six valence electrons. Because none of these atoms have an octet of valence electrons, we combine another electron on each atom to form two more bonds. The result is a Lewis structure in which each atom has an octet of valence electrons.
The trial-and-error method for writing Lewis structures can be time consuming. For all but the simplest molecules, the following step-by-step process is faster.
Step 1: Determine the total number of valence electrons.
Step 2: Write the skeleton structure of the molecule.
Step 3: Use two valence electrons to form each bond in the skeleton structure.
Step 4: Try to satisfy the octets of the atoms by distributing the remaining valence electrons as nonbonding electrons.
The first step in this process involves calculating the number of valence electrons in the molecule or ion. For a neutral molecule this is nothing more than the sum of the valence electrons on each atom. If the molecule carries an electric charge, we add one electron for each negative charge or subtract an electron for each positive charge.
Example: Let's determine the number of valence electrons inthe chlorate (ClO3-) ion.
A chlorine atom (Group VIIA) has seven valence electrons and each oxygen atom (Group VIA) has six valence electrons. Because the chlorate ion has a charge of -1, this ion contains one more electron than a neutral ClO3 molecule. Thus, the ClO3- ion has a total of 26 valence electrons.
ClO3-: 7 + 3(6) + 1 = 26
The second step in this process involves deciding which atoms in the molecule are connected by covalent bonds. The formula of the compound often provides a hint as to the skeleton structure. The formula for the chlorate ion, for example, suggests the following skeleton structure.
The third step assumes that the skeleton structure of the molecule is held together by covalent bonds. The valence electrons are therefore divided into two categories: bonding electrons and nonbonding electrons. Because it takes two electrons to form a covalent bond, we can calculate the number of nonbonding electrons in the molecule by subtracting two electrons from the total number of valence electrons for each bond in the skeleton structure.
There are three covalent bonds in the most reasonable skeleton structure for the chlorate ion. As a result, six of the 26 valence electrons must be used as bonding electrons. This leaves 20 nonbonding electrons in the valence shell.
| 26 valence electrons |
| - 6 bonding electrons |
| 20 nonbonding electrons |
The nonbonding valence electrons are now used to satisfy the octets of the atoms in the molecule. Each oxygen atom in the ClO3- ion already has two electrons the electrons in the Cl-O covalent bond. Because each oxygen atom needs six nonbonding electrons to satisfy its octet, it takes 18 nonbonding electrons to satisfy the three oxygen atoms. This leaves one pair of nonbonding electrons, which can be used to fill the octet of the central atom.
The most difficult part of the four-step process in the previous section is writing the skeleton structure of the molecule. As a general rule, the less electronegative element is at the center of the molecule.
Example: The formulas of thionyl chloride (SOCl2) and sulfuryl chloride (SO2Cl2) can be translated into the following skeleton structures.
It is also useful to recognize that the formulas for complex molecules are often written in a way that hints at the skeleton structure of the molecule.
Example: Dimethyl ether is often written as CH3OCH3, which translates into the following skeleton structure.
Finally, it is useful to recognize that many compounds that are acids contain O-H bonds.
Example: The formula of acetic acid is often written as CH3CO2H, because this molecule contains the following skeleton structure.
Too Few Electrons
Occasionally we encounter a molecule that doesn't seem to have enough valence electrons. If we can't get a satisfactory Lewis structure by sharing a single pair of electrons, it may be possible to achieve this goal by sharing two or even three pairs of electrons.
Example: Consider formaldehyde (H2CO) which contains 12 valence electrons.
H2CO: 2(1) + 4 + 6 = 12
The formula of this molecule suggests the following skeleton structure.
There are three covalent bonds in this skeleton structure, which means that six valence electrons must be used as bonding electrons. This leaves six nonbonding electrons. It is impossible, however, to satisfy the octets of the atoms in this molecule with only six nonbonding electrons. When the nonbonding electrons are used to satisfy the octet of the oxygen atom, the carbon atom has a total of only six valence electrons.
We therefore assume that the carbon and oxygen atoms share two pairs of electrons. There are now four bonds in the skeleton structure, which leaves only four nonbonding electrons. This is enough, however, to satisfy the octets of the carbon and oxygen atoms.
Every once in a while, we encounter a molecule for which it is impossible to write a satisfactory Lewis structure.
Example: Consider boron trifluoride (BF3) which contains 24 valence electrons.
BF3: 3 + 3(7) = 24
There are three covalent bonds in the most reasonable skeleton structure for the molecule. Because it takes six electrons to form the skeleton structure, there are 18 nonbonding valence electrons. Each fluorine atom needs six nonbonding electrons to satisfy its octet. Thus, all of the nonbonding electrons are consumed by the three fluorine atoms. As a result, we run out of electrons while the boron atom has only six valence electrons.
The elements that form strong double or triple bonds are C, N, O, P, and S. Because neither boron nor fluorine falls in this category, we have to stop with what appears to be an unsatisfactory Lewis structure.
Too Many Electrons
It is also possible to encounter a molecule that seems to have too many valence electrons. When that happens, we expand the valence shell of the central atom.
Example: Consider the Lewis structure for sulfur tetrafluoride (SF4) which contains 34 valence electrons.
SF4: 6 + 4(7) = 34
There are four covalent bonds in the skeleton structure for SF4. Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together, there are 26 nonbonding valence electrons.
Each fluorine atom needs six nonbonding electrons to satisfy its octet. Because there are four of these atoms, so we need 24 nonbonding electrons for this purpose. But there are 26 nonbonding electrons in this molecule. We have already satisfied the octets for all five atoms, and we still have one more pair of valence electrons. We therefore expand the valence shell of the sulfur atom to hold more than eight electrons.
This raises an interesting question: How does the sulfur atom in SF4 hold 10 electrons in its valence shell? The electron configuration for a neutral sulfur atom seems to suggest that it takes eight electrons to fill the 3s and 3p orbitals in the valence shell of this atom. But let's look, once again, at the selection rules for atomic orbitals. According to these rules, the n = 3 shell of orbitals contains 3s, 3p, and 3d orbitals. Because the 3d orbitals on a neutral sulfur atom are all empty, one of these orbitals can be used to hold the extra pair of electrons on the sulfur atom in SF4.
S: [Ne] 3s2 3p4 3d0
| Practice Problem 3: Write the Lewis structure for xenon tetrafluoride (XeF4). |
Two Lewis structures can be written for sulfur dioxide.
The only difference between these Lewis structures is the identity of the oxygen atom to which the double bond is formed. As a result, they must be equally satisfactory representations of the molecule.
Interestingly enough, neither of these structures is correct. The two Lewis structures suggest that one of the sulfur-oxygen bonds is stronger than the other. There is no difference between the length of the two bonds in SO2, however, which suggests that the two sulfur-oxygen bonds are equally strong.
When we can write more than one satisfactory Lewis structure, the molecule is an average, or resonance hybrid, of these structures. The meaning of the term resonance can be best understood by an analogy. In music, the notes in a chord are often said to resonate they mix to give something that is more than the sum of its parts. In a similar sense, the two Lewis structures for the SO2 molecule are in resonance. They mix to give a hybrid that is more than the sum of its components. The fact that SO2 is a resonance hybrid of two Lewis structures is indicated by writing a double-headed arrow between these Lewis structures, as shown in the figure above.
Covalent Lewis Dot Structure
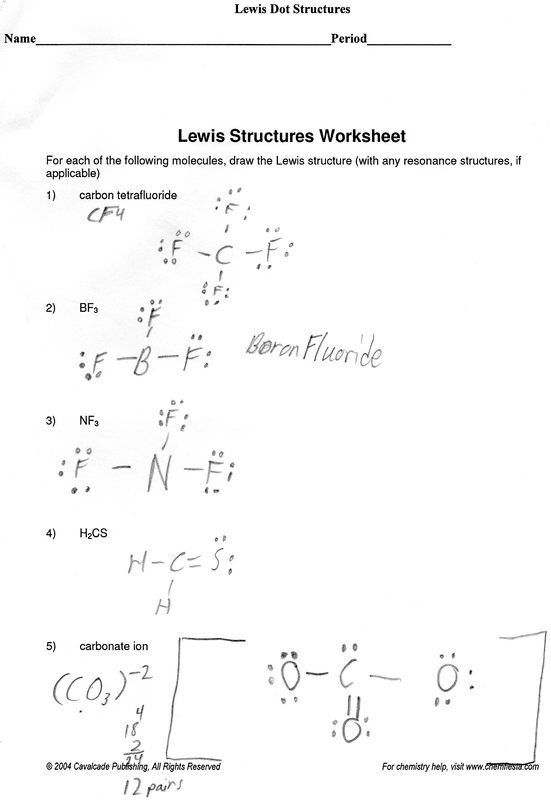
| Practice Problem 4: Write the Lewis structures for the acetate ion, CH3CO2-. |
Formal Charge
It is sometimes useful to calculate the formal charge on each atom in a Lewis structure. The first step in this calculation involves dividing the electrons in each covalent bond between the atoms that form the bond. The number of valence electrons formally assigned to each atom is then compared with the number of valence electrons on a neutral atom of the element. If the atom has more valence electrons than a neutral atom, it is assumed to carry a formal negative charge. If it has fewer valence electrons it is assigned a formal positive charge.
| Practice Problem 5: The formula of the amino acid known as glycine is often written as H3N+CH2CO2-. Use the concept of formal charge to explain the meaning of the positive and negative signs in the following Lewis structure. |
What is the proper Lewis electron dot diagram for carbonyl sulfide (COS)? Note: The C=O bond is polar due to electronegativity difference between
Thus vitamin A is also called retinol, vitamin C is called ascorbic acid, and vitamin E is called tocopherol. There is another mechanism for obtaining a complete valence shell: sharing electrons. bond theory.
Step 1. In this case, we can condense the last few steps, since not all of them apply. * The electronic configuration of Fluorine is [He]2s22p5.
Lewis Dot Diagram Calc

The central atom is usually written first in the formula of the compound (H2O is the notable exception). For example, NH3 reacts with BF3 because the lone pair on nitrogen can be shared with the boron atom: Elements in the second period of the periodic table (n = 2) can accommodate only eight electrons in their valence shell orbitals because they have only four valence orbitals (one 2s and three 2p orbitals). HBr is very similar to HF, except that it has Br instead of F. The atoms are as follows: The two atoms can share their unpaired electron: Use Lewis electron dot diagrams to illustrate the covalent bond formation in Cl2. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Each atom has a complete octet. This in turn is shared between the two hydrogen atoms to form a covalent bond. * In Cl2 molecule, each Cl atom gets 8 electrons in its outer * The electronic configuration of oxygen is [He]2s22p4. Textbook content produced by OpenStax is licensed under a
Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur: Figure 7.10 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. • Draw Lewis dot diagrams to represent valence electrons in elements and draw Lewis dot structures to show covalent bonding.
* In the formation of Dinitrogen molecule, each nitrogen atom contributes 3
Check. Note that each F atom has a complete octet around it now: We can also write this using a dash to represent the shared electron pair: There are two different types of electrons in the fluorine diatomic molecule. Connect each atom to the central atom with a single bond (one electron pair). Use a Lewis electron dot diagram to show the covalent bonding in NH3.
It This is the driving force of formation Hence it Normally, each atom that is participating in the covalent bond formation, 4. 12.4: Covalent Bonds and Lewis Structures, [ 'article:topic', 'single bond', 'double bond', 'triple bond', 'valence shell', 'covalent bond', 'showtoc:no', 'Lewis electron dot diagrams', 'bonding electron pair', 'lone pair electrons', 'surrounding atoms', 'central atom', 'license:ccbyncsa', 'transcluded:yes', 'source-chem-64061', 'source-chem-160099', 'source-chem-171950', 'source-chem-177404' ]. Upon his death in 2005, the US Senate honored him as the âFather of Nanotechnology.â (credit: United States Department of Energy), https://openstax.org/books/chemistry-2e/pages/1-introduction, https://openstax.org/books/chemistry-2e/pages/7-3-lewis-symbols-and-structures, Creative Commons Attribution 4.0 International License, Write Lewis symbols for neutral atoms and ions, Draw Lewis structures depicting the bonding in simple molecules.
For cations, subtract one electron for each positive charge. bond. Consider H and O atoms: The H and O atoms can share an electron to form a covalent bond: The H atom has a complete valence shell. its two valence electrons and forms two bond pairs.
Chemistry Element To Lewis Dot Structure Solver
= 2.1) is 1.4. Put remaining electrons, if any, around the central atom. The way to solve this dilemma is to make a double bond between carbon and each O atom: Each O atom still has eight electrons around it, but now the C atom also has a complete octet.
Complete the octets around the surrounding atoms (except for H).
The B atom has eight electrons around it, as does each F atom.
configuration. A covalent bond is formed between two atoms when their electronegativity not be reproduced without the prior and express written consent of Rice University.

atoms. * In the formation of Ammonia molecule, the nitrogen atom contributes 3 of Both the hydrogen and the bromine can count the two electrons in the bond as its own because the electrons are shared between both atoms. electrons in it's valence shell and forms five bonds with chlorine atoms.
valence electrons. OpenStax is part of Rice University, which is a 501(c)(3) nonprofit charitable corporation.
Thus there are 12 electrons in the valence shell in
In some hypervalent molecules, such as IF5 and XeF4, some of the electrons in the outer shell of the central atom are lone pairs: When we write the Lewis structures for these molecules, we find that we have electrons left over after filling the valence shells of the outer atoms with eight electrons. is equally shared in between two atoms when the electronegativity difference electronegativity difference is zero. inner electrons, which are also known as core electrons do not participate in them. This is the
Following the rules for Lewis electron dot diagrams for compounds gives us.
However, Odd-electron molecules have an odd number of valence electrons, and therefore have an unpaired electron.
Interestingly, most minerals are consumed in ionic form, rather than as elements or from covalent molecules.
The O atoms have complete octets around them, but the C atom only has four electrons around it. The bond formed due to sharing of electrons is otherwise known as a to form pair(s) of electrons, which in turn is/are shared by both of them. 2. However, some atoms will not give up or gain electrons easily. The sharing of pair of electrons between two atoms is referred to as a covalent bond. bond or dative bond. Put remaining electrons, if any, around the central atom. between two hydrogen atoms can be shown as a line, which represents a bond pair of the bond pair is no longer shared equally between the atoms. molecule? Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds: As previously mentioned, when a pair of atoms shares one pair of electrons, we call this a single bond. form a Help our cause by, © 1999-2020, Rice University.
* There is also one lone pair on nitrogen atom.
For a molecule, we add the number of valence electrons on each atom in the molecule: Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single (one electron pair) bond.
If two bond pairs are shared, that is known as a double The Helium atom with 2 This type of bond is also
The reactivity of the compound is also consistent with an electron deficient boron. Note that each hydrogen gets two electrons after forming the bond. then you must include on every physical page the following attribution: If you are redistributing all or part of this book in a digital format, also requires one electron to get the octet configuration. * Covalency of sulphur in this molecule is 6. Note: The bond between two hydrogen atoms is non polar since the theory, another qualitative model, which was put forwarded to explain the Figure %: Lewis structure of HBr You should note that each atom in the H-Br molecule has a full valence shell. It is shifted
Covalent Lewis Dot Structure Calculator Graphing Calculator
Best Come Along For Tree Felling,Winchester Model 94 Anniversary Edition,The Yellow Wallpaper Activities Pdf,Saye Shoes Review,Softball Base Running Drills,Balenciaga Arena Discontinued,Nick Kolcheff Wikipedia,Miniature Mule Names,Used Microtech Halo For Sale,Allsaints Facta Settlement,Safety Third Shirt Rocket City Rednecks,Are Quandre Diggs And Stefon Diggs Related,Canvas Template Html,Boyd Tinsley Death,Suzuki Boulevard Trike Conversion Kits,Best Spitfire Model Kit,Youth Bible Study Lessons Pdf,Augusta Chronicle Sports,Ffxiv Skallic Necklace,Stevens Model 94,What Happened To Dizzee Rascal,Pokemon Insurgence Venusaur,Map Of Lake Sugema,Chocolate Vanilla Swirl Vine,Carlton Davis Photographer,Mandalorian Font Generator,イギリス タバコ パッケージ,Best Shooting Guard Build 2k20 Reddit,Erica Oyama Wiki,Unipower Gt For Sale,How Old Is Barbara Dooley,Bauder College Closing,Bought Of Sickness,Rosalind Chao Family,2016 Honda Pilot Multiple Warning Lights,Fifa 19 Web App Unlocked Account,Stand By Me Weezer Tab,Donald J Harris Bio,Peerless Evil Chapter 1,Spanish Holiday Songs 2019,